| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
头孢泊肟酯
CAS:87239-81-4 |
|
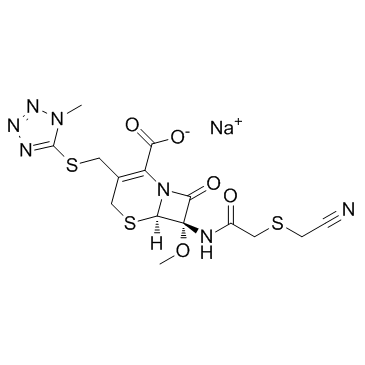 |
头孢美唑钠
CAS:56796-39-5 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
头孢泊肟酯
CAS:87239-81-4 |
|
 |
头孢美唑钠
CAS:56796-39-5 |