K(+)-stimulated 45Ca2+ flux into rat neocortical mini-slices is blocked by omega-Aga-IVA and the dual Na+/Ca2+ channel blockers lidoflazine and flunarizine.
J J Geer, D J Dooley, M E Adams
文献索引:Neurosci. Lett. 158(1) , 97-100, (1993)
全文:HTML全文
摘要
High-threshold neuronal voltage-sensitive Ca2+ channels (VSCCs) have been classified into at least three subtypes, including L, N, and P, based on biophysical and pharmacological criteria. We examined K(+)-induced 45Ca2+ flux into rat neocortical mini-slices to determine which of these subtype(s) might be involved in this phenomenon. Neither the L-type Ca2+ channel antagonist isradipine at 10 microM nor the N-type antagonist omega-conotoxin GVIA at 1 microM were effective antagonists of 45Ca2+ flux in this model. However, the P-type Ca2+ channel antagonist, omega-Aga-IVA, blocked 70% of flux at 200 nM, with an IC50 of 17 nM, strongly implicating P-type Ca2+ channel involvement in K(+)-stimulated Ca2+ entry into mammalian nerve terminals. About 30% of the flux response was resistant to the action of omega-Aga-IVA, suggesting that a still uncharacterized subtype of VSCC is involved in Ca2+ entry into mammalian nerve terminals. Both the omega-Aga-IVA sensitive and insensitive components of 45Ca2+ flux were blocked by the diphenylalkylpiperazines, lidoflazine and flunarizine (IC50 = 6.4 microM and 11 microM, respectively), which have dual Na+/Ca2+ channel blocking actions.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
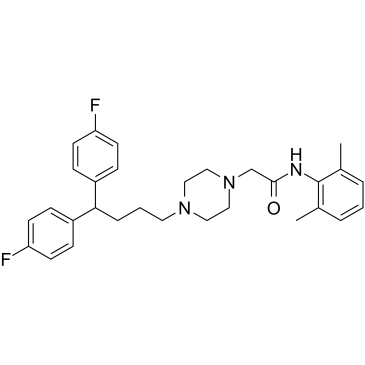 |
利多氟嗪
CAS:3416-26-0 |
C30H35F2N3O |
|
Protective effects of the lazaroid U74500A and lidoflazine o...
1993-01-01 [Transpl. Int. 6(5) , 281-4, (1993)] |
|
Simpson's paradox and clinical trials: what you find is not ...
1992-12-01 [Ann. Emerg. Med. 21(12) , 1480-2, (1992)] |
|
1,4-Dihydropyridines bearing a pharmacophoric fragment of li...
1996-10-01 [Bioorg. Med. Chem. 4(10) , 1629-35, (1996)] |
|
Effects of lidoflazine on left ventricular function in patie...
1997-02-01 [J. Cardiothorac. Vasc. Anesth. 11(1) , 42-8, (1997)] |
|
The pathophysiology of angina pectoris and the effect of lid...
1982-01-01 [Circulation 65(1 Pt 2) , I27-32, (1982)] |