High-performance liquid chromatographic enantioseparation of unusual isoxazoline-fused 2-aminocyclopentanecarboxylic acids on (+)-(18-crown-6)-2,3,11,12-tetracarboxylic acid-based chiral stationary phases.
László Sipos, István Ilisz, Anita Aranyi, Zsanett Gecse, Melinda Nonn, Ferenc Fülöp, Myung Ho Hyun, Antal Péter
文献索引:Chirality 24(10) , 817-24, (2012)
全文:HTML全文
摘要
The enantiomers of four unusual isoxazoline-fused 2-aminocyclopentanecarboxylic acids were directly separated on chiral stationary phases containing (+)-(18-crown-6)-2,3,11,12-tetracarboxylic acid as chiral selector. The nature of the alcoholic modifier (MeOH, EtOH, IPA) exerted a great effect on the retention, whereas the selectivity and resolution did not change substantially. Two types of dependence of retention on alcohol content were detected: k(1) increased continuously with increasing alcohol content or a U-shaped retention curve was observed. A comparison of the chromatographic data obtained with HCOOH, AcOH, TFA, HClO(4), H(2)SO(4), or H(3)PO(4) as acidic modifier at a constant concentration demonstrated that in most cases, larger k values were obtained on the application of AcOH or HCOOH, and an increase of the acid content resulted in a decrease of retention. Some mechanistic aspects of the chiral recognition process are discussed with respect to the structures of the analytes and selector. The sequence of elution of the enantiomers was determined in all cases.Copyright © 2012 Wiley Periodicals, Inc.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
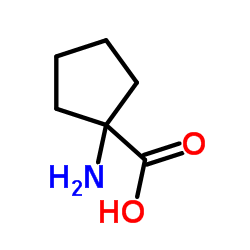 |
环亮氨酸
CAS:52-52-8 |
C6H11NO2 |
|
Quantitative structure-activity relationship and complex net...
2008-11-13 [J. Med. Chem. 51 , 6740-51, (2008)] |
|
In vivo stimulus-induced vasodilation occurs without IP3 rec...
2013-05-08 [J. Neurosci. 33(19) , 8411-22, (2013)] |
|
Drug design, in vitro pharmacology, and structure-activity r...
2009-08-27 [J. Med. Chem. 52 , 5093-107, (2009)] |
|
Quantal amplitude at the cone ribbon synapse can be adjusted...
2011-01-01 [Mol. Vis. 17 , 920-31, (2011)] |
|
Conformations of peptides containing a chiral cyclic α, α-di...
2010-11-01 [J. Pept. Sci. 16(11) , 621-6, (2010)] |