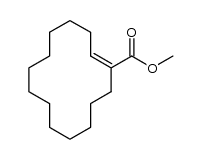
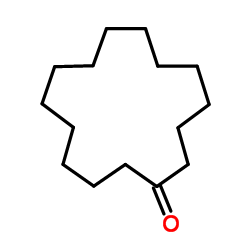
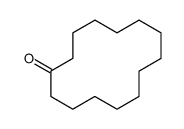
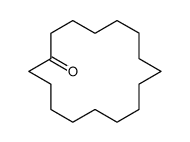
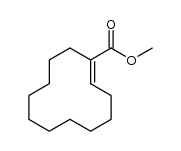
|
~89% |
|
~32% |
|
~48% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
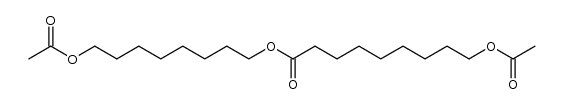
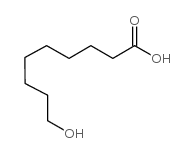

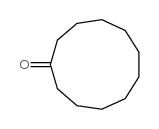


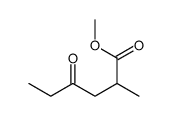
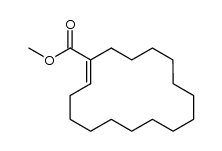
![1-oxaspiro[2.11]tetradecane结构式](https://image.chemsrc.com/caspic/347/33059-17-5.png)