A synthesis of heteroaromatic analogues of 1-methyl-1,2,3,4-tetrahydroisoquinoline using the Pummerer-type cyclization reaction: observation of tandem cyclization reaction.
Yoshie Horiguchi, Keita Ogawa, Toshiaki Saitoh, Takehiro Sano
文献索引:Chem. Pharm. Bull. 52(2) , 214-20, (2004)
全文:HTML全文
摘要
The sulfoxides 7b and 7d carrying thiophene or benzothiophene as heteroaromatic nucleophiles, when treated with trifluoroacetic anhydride at room temperature (Pummerer reaction), underwent an intramolecular alkylation in an exclusive manner to yield 4,5,6,7-tetrahydro-7-methyl-4-phenylsulfanylthieno[2,3-c]pyridine-6-carbaldehyde (10) and the corresponding benzothiophene derivative (12b) in high yields, respectively. Thus, this route provides biologically interesting nitrogen heterocycles (1b) and (2b). On the other hand, the sulfoxide (7c) carrying benzofuran as a nucleophile on reaction with TFAA yielded not only the Pummerer-type cyclization product (12a), but also the diastereoisomeric tandem cyclization products (13) and (14) having a noble 11-aza-2-oxa-7-thiatricyclo[4.3.3.0(1,5)]dodecane ring system (B). The formation of these products can be readily rationalized by the intervention of the oxonium ion intermediate (21).
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
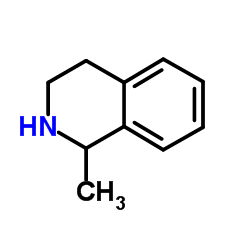 |
1-甲基-1,2,3,4-四氢异喹啉
CAS:4965-09-7 |
C10H13N |
|
Comparative behavioral and neurochemical studies of R- and S...
2012-01-01 [Pharmacol. Rep. 64(4) , 857-69, (2012)] |
|
Important role of 3-methoxytyramine in the inhibition of coc...
2010-01-01 [Pharmacol. Rep. 62(6) , 983-97, (2010)] |
|
Determination method of 1-methyl-1,2,3, 4-tetrahydroisoquino...
2002-05-03 [Life Sci. 70(24) , 2871-83, (2002)] |
|
Interactions of 1-methyl-1,2,3,4-tetrahydroisoquinoline with...
2010-01-01 [Epilepsy Res. 89(2-3) , 207-19, (2010)] |
|
The interaction of tetrahydroisoquinoline derivatives with a...
2003-11-01 [J. Neural Transm. Gen. Sect. 110(11) , 1205-13, (2003)] |