Laccase-mediated formation of the phenoxazinone derivative, cinnabarinic acid.
C Eggert, U Temp, J F Dean, K E Eriksson
文献索引:FEBS Lett. 376(3) , 202-6, (1995)
全文:HTML全文
摘要
The phenoxazinone chromophore occurs in a variety of biological systems, including numerous pigments and certain antibiotics. It also appears to form as part of a mechanism to protect mammalian tissue from oxidative damage. During cultivation of the basidiomycete, Pycnoporus cinnabarinus, a red pigment was observed to accumulate in the culture medium. It was identified as the phenoxazinone derivative, cinnabarinic acid (CA). Laccase was the predominant extracellular phenoloxidase activity in P. cinnabarinus cultures. In vitro studies showed that CA was formed after oxidation of the precursor, 3-hydroxyanthranilic acid (3-HAA), by laccases. Moreover, oxidative coupling of 3-HAA to form CA was also demonstrated for the mammalian counterpart of laccase, the blue copper oxidase, ceruloplasmin.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
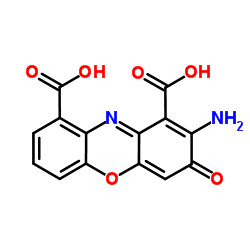 |
朱砂精酸
CAS:606-59-7 |
C14H8N2O6 |
|
Aminophenoxazinones as inhibitors of indoleamine 2,3-dioxyge...
2013-04-25 [J. Med. Chem. 56(8) , 3310-7, (2013)] |
|
Simultaneous determination of 3-hydroxyanthranilic and cinna...
1992-02-01 [Anal. Biochem. 200(2) , 273-9, (1992)] |
|
Oxidation of 3-hydroxyanthranilic acid to the phenoxazinone ...
1992-09-01 [Biochemistry 31(34) , 8090-7, (1992)] |
|
Mechanism of reaction of 3-hydroxyanthranilic acid with mole...
1990-05-16 [Biochim. Biophys. Acta 1034(2) , 207-12, (1990)] |
|
Oxidative reactivity of the tryptophan metabolites 3-hydroxy...
1987-01-15 [Biochem. Pharmacol. 36(2) , 211-7, (1987)] |