| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
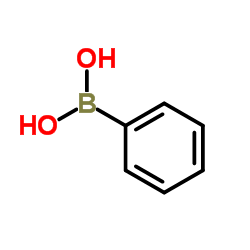 |
苯硼酸
CAS:98-80-6 |
|
 |
3-(三氟甲基)苯硼酸
CAS:1423-26-3 |
|
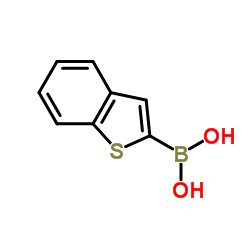 |
苯并噻吩-2-硼酸
CAS:98437-23-1 |
|
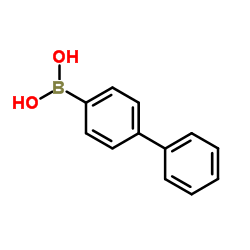 |
4-联苯硼酸
CAS:5122-94-1 |
|
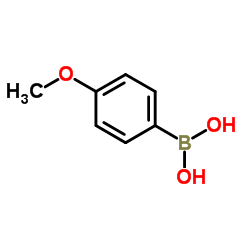 |
4-甲氧基苯硼酸
CAS:5720-07-0 |