Analytical Biochemistry
1987-08-15
2,3-Diaminophenazine is the product from the horseradish peroxidase-catalyzed oxidation of o-phenylenediamine.
P J Tarcha, V P Chu, D Whittern
文献索引:Anal. Biochem. 165 , 230, (1987)
全文:HTML全文
摘要
NMR and mass spectroscopic evidence has been obtained which indicates that the product of the oxidation of o-phenylenediamine by hydrogen peroxide, uncatalyzed or catalyzed by horseradish peroxidase, is 2,3-diaminophenazine. These results settle disparate literature descriptions. The process is most likely free radical in nature starting with the abstraction of a labile amino hydrogen atom.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
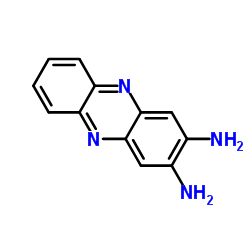 |
2,3-二氨基酚嗪
CAS:655-86-7 |
C12H10N4 |
相关文献:
更多...
|
Spectrophotometric quantification of horseradish peroxidase ...
2010-12-15 [Anal. Biochem. 407(2) , 293-5, (2010)] |
|
Spectrophotometric quantification of lactose in solution wit...
2011-10-01 [Anal. Bioanal. Chem 401 , 2307-2310, (2011)] |
|
Photoinduced DNA cleavage and cellular damage in human derma...
2005-01-01 [Photochem. Photobiol. 81(1) , 89-95, (2005)] |
|
Experimental (FT-IR, FT-Raman and UV-Vis) spectra and theore...
2012-10-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 96 , 401-12, (2012)] |
|
Studies on the genotoxic effects of aminophenazines using tw...
1999-10-29 [Mutat. Res. 446(1) , 57-65, (1999)] |