Site-specific tetrameric streptavidin-protein conjugation using sortase A.
Takuya Matsumoto, Shiori Sawamoto, Takayuki Sakamoto, Tsutomu Tanaka, Hideki Fukuda, Akihiko Kondo
文献索引:J. Biotechnol. 152 , 37-42, (2011)
全文:HTML全文
摘要
Streptavidin is tetrameric protein which has tight and specific biotin binding affinity, and streptavidin modification of proteins or small molecules is widely used for biotechnology tool. Here, we demonstrate site-specific streptavidin-protein conjugation using enzymes. We focused on sortase A, a transpeptidase from Staphylococcus aureus. A streptavidin-tagged LPETG motif (Stav-LPETG) was expressed in Escherichia coli. We achieved soluble streptavidin expression in E. coli without refolding using a cold shock expression system. Then we successfully conjugated Stav-LPETG with pentaglycine-appended green fluorescence protein (Gly5-GFP) or triglycine-appended glucose oxidase (Gly3-GOD) using sortase A. SDS-PAGE analysis showed site-specific tetrameric streptavidin-protein conjugation with the tagged proteins. In addition, the functions of a Stav-GOD conjugate, i.e., biotin-binding and glucose oxidase activity, were significantly higher compared to those of streptavidin-GOD conjugates prepared by chemical modification.Copyright © 2011 Elsevier B.V. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
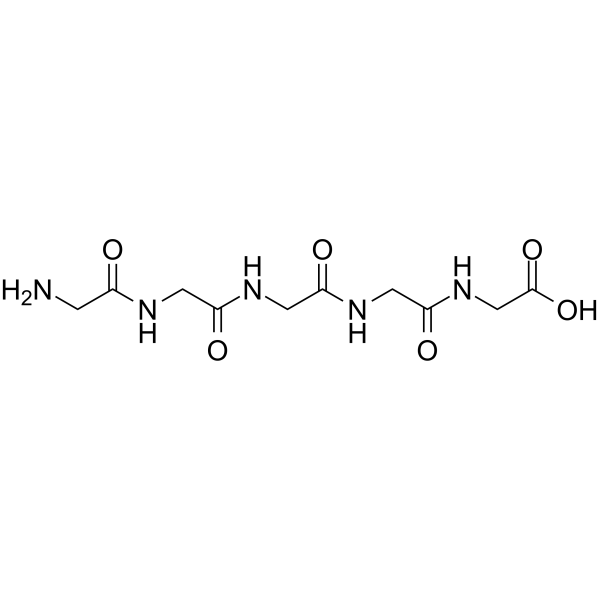 |
五甘氨酸
CAS:7093-67-6 |
C10H17N5O6 |
|
Peptidoglycan crosslinking relaxation plays an important rol...
2011-01-01 [PLoS ONE 6 , e17054, (2011)] |
|
Modeling the electrophoresis of oligoglycines.
2010-08-01 [J. Sep. Sci. 33 , 2430-2438, (2010)] |
|
[Mode of action of microbial anti-MRSA agents].
2012-01-01 [Yakugaku Zasshi 132 , 37-44, (2012)] |
|
Carnosine retards tumor growth in vivo in an NIH3T3-HER2/neu...
2010-01-01 [Mol. Cancer 9 , 2, (2010)] |
|
The Staphylococcus aureus peptidoglycan protects mice agains...
2011-01-01 [PLoS ONE 6 , e28377, (2011)] |