| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
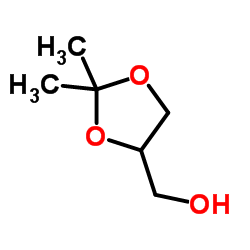 |
丙酮缩甘油
CAS:100-79-8 |
|
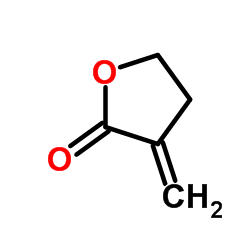 |
α-亚甲基-γ-丁内酯
CAS:547-65-9 |
|
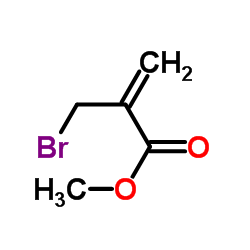 |
2-溴甲基丙烯酸甲酯
CAS:4224-69-5 |