| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
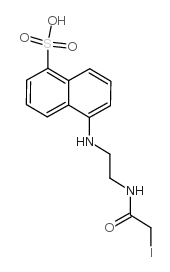 |
N-碘乙酰-N'-(5-磺基-1-萘)乙二胺
CAS:36930-63-9 |
|
 |
羧苄青霉素钠
CAS:4800-94-6 |