Characterization of low density lipoprotein receptor ligand interactions by fluorescence resonance energy transfer.
Taichi Yamamoto, Johanne Lamoureux, Robert O Ryan
文献索引:J. Lipid Res. 47(5) , 1091-6, (2006)
全文:HTML全文
摘要
The low density lipoprotein receptor (LDLR) is the prototype of a family of cell surface receptors involved in a wide range of biological processes. A soluble low density lipoprotein receptor (sLDLR) and a tryptophan (Trp)-deficient variant human apolipoprotein E3 (apoE3) N-terminal domain (NT) were used in binding studies. The sole cysteine in apoE3-NT was covalently modified with an extrinsic fluorescence probe, N-(iodoacetyl)-N'-(5-sulfo-1-napthyl)ethylenediamine (AEDANS), and the protein was complexed with lipid. Incubation of sLDLR with AEDANS-Trp-null apoE3-NT dimyristoylphosphatidylcholine (DMPC) disks, but not lipid-free AEDANS-apoE, induced an enhancement in AEDANS fluorescence emission intensity (excitation, 280 nm) consistent with intermolecular energy transfer from excited Trp in sLDLR to receptor-bound apoE. Ligand binding to sLDLR required calcium and was saturable. In competition binding assays, unlabeled apoE3-NT DMPC inhibited AEDANS-apoE DMPC binding to sLDLR more effectively than low density lipoprotein. Fluorescence changes in this system reflected pH-dependent ligand binding and release from sLDLR consistent with models derived from the X-ray crystal structure of the receptor at endosomal pH. Intermolecular energy transfer from excited Trp in LDLR family members to fluorescently tagged ligands represents a sensitive and convenient assay for the characterization of the myriad molecular interactions ascribed to this family of receptor.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
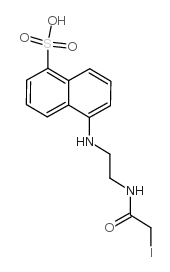 |
N-碘乙酰-N'-(5-磺基-1-萘)乙二胺
CAS:36930-63-9 |
C14H15IN2O4S |
|
Detection of proteins on blot membranes.
2001-05-01 [Curr. Protoc. Protein Sci. Chapter 10 , Unit 10.8, (2001)] |
|
Iron-sulfur cluster biosynthesis: characterization of IscU-I...
2009-08-01 [J. Biol. Inorg. Chem. 14(6) , 829-39, (2009)] |
|
Characterization of the formation of amyloid protofibrils fr...
2006-05-12 [J. Mol. Biol. 358(4) , 935-42, (2006)] |
|
Fluorescence-based peptide labeling and fractionation strate...
2005-07-15 [Anal. Chem. 77(14) , 4495-502, (2005)] |
|
Decavanadate binding to a high affinity site near the myosin...
2004-05-11 [Biochemistry 43(18) , 5551-61, (2004)] |