Liver cirrhosis with ascites: pathogenesis of resistance to diuretics and long-term efficacy and safety of torasemide.
H Knauf, E Mutschler
文献索引:Cardiology 84 Suppl 2 , 87-98, (1994)
全文:HTML全文
摘要
In a prospective randomized short-term study, the efficacy and safety of xipamide and a combination of spironolactone and furosemide were compared in the treatment of hepatic cirrhotic ascites. Twenty-two patients were randomized to either xipamide, 20 mg/day (group I), or spironolactone, 200 mg/day, combined with furosemide, 40 mg on alternate days (group II). During the first 4 days of treatment, adequate diuresis, measured as loss of body weight greater than 1.6 kg, occurred in 7 patients in group I and in 3 in group II. In the latter group, another 4 patients responded satisfactorily after a further 4 days of treatment. Four patients in group I who failed to respond to xipamide with an adequate loss of body weight were subsequently treated with the spironolactone-furosemide combination, but only one responded. Two patients in group II who failed to respond to the combination of spironolactone and furosemide also failed to respond to xipamide. In both groups, a positive diuretic response occurred only when the pretreatment fractional sodium excretion exceeded 0.2%. Diuretic resistance was overcome only by additional treatments which reduced proximal tubular sodium reabsorption. Xipamide commonly induced hypokalaemia; in contrast hyperkalaemia was seen following treatment with the spironolactone-furosemide combination. Renal function remained stable in all patients during both diuretic treatments. An open ongoing 6-month trial of torasemide, 10-40 mg/day, in combination with spironolactone, 50-400 mg/day, has also been undertaken in 117 patients with cirrhotic ascites who showed inadequate responses to salt and water restriction and spironolactone alone. Twenty-seven patients have been withdrawn from the study, 9 for the complications of hepatic coma, bleeding oesophageal varices, or hyponatraemia. Twenty-two patients are still being treated and 68 have completed the trial on a mean dose of torasemide, 15 mg/day. Body weight was reduced by a mean of 2.3 kg at 6 weeks, 2.6 kg at 14 weeks and 3.2 kg after 6 months. Loss of body weight was primarily associated with reduction of ascites and secondarily with reduction of peripheral oedema. There were no untoward adverse reactions with torasemide, and no significant changes in serum electrolytes, liver, renal, or haematological variables.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
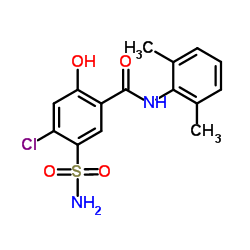 |
希伯胺
CAS:14293-44-8 |
C15H15ClN2O4S |
|
Clinicopharmacological reappraisal of the potency of diureti...
1993-01-01 [Cardiovasc. Drugs Ther. 7 Suppl 1 , 23-8, (1993)] |
|
Bioavailability study of triamterene and xipamide using urin...
2012-03-05 [J. Pharm. Biomed. Anal. 61 , 78-85, (2012)] |
|
Spectrophotometric and spectrodensitometric determination of...
2010-03-01 [Drug Test. Anal. 2(3) , 113-21, (2010)] |
|
Selective and sensitive spectrophotometric method for the de...
2012-11-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 97 , 284-9, (2012)] |
|
Can xipamide or tacrolimus inhibit the glucuronidation of my...
2003-06-01 [Exp. Toxicol. Pathol. 54(5-6) , 375-9, (2003)] |