An unexpected interaction between the modular polyketide synthases, erythromycin DEBS1 and pikromycin PikAIV, leads to efficient triketide lactone synthesis.
Beom Seok Kim, T Ashton Cropp, Galina Florova, Yuko Lindsay, David H Sherman, Kevin A Reynolds
文献索引:Biochemistry 41(35) , 10827-33, (2002)
全文:HTML全文
摘要
An unusual feature of the 6-module pikromycin polyketide synthase (PikPKS, PikAI-PikAIV) of S. venezuelae is the ability to generate both 12- and 14-membered ring macrolides. The PikAIV component containing the last extension module and a thioesterase domain is responsible for generating both of these products. In the case of the 12-membered ring macrolide, an acyl-enzyme intermediate on PikAIII is able to efficiently "skip" the last extension step and is cyclized by the TE domain of PikAIV, presumably as a result of a PikAIII-PikAIV interaction. Herein we report that plasmid-based expression (pBK3) of DEBS1, which comprises the loading domain and the first two modules of the Saccharopolyspora erythrea 6-deoxyerythronolide B synthase, in S. venezuelae leads to efficient 15 +/- 3 mg/L production of triketide lactone products (TKLs). Comparable levels of TKLs were observed with a plasmid (pBK1) which expressed DEBS1 fused to a TE domain (DEBS1-TE). These results are in stark contrast to previous in vivo and in vitro analyses, where only DEBS1-TE efficiently produces TKLs. Levels of TKLs decreased dramatically with expression of DEBS1 in both pikAIV and pikAIII-pikAIV deletion hosts (0.5 mg/L), but not DEBS1-TE, and could be partially restored by addition of a PikAIV complementation plasmid. These data suggest that PikAIV is able to efficiently catalyze formation of 6-membered lactone ring products from acyl-bound intermediates on DEBS1 in a manner analogous to that observed for 12-membered macrolide products from PikAIII. Significant sequence similarity and length of the C-terminal linker region of PikAIII and DEBS1 suggest that this region may be responsible for the interaction with PikAIV. A replacement of this linker region of DEBS1 with the corresponding region of PikAI led to a 95% decrease in TKL levels in S. venezuelae, consistent with this hypothesis.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
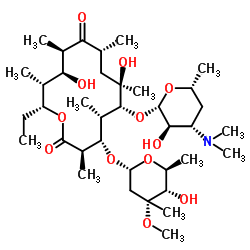 |
红霉素B
CAS:527-75-3 |
C37H67NO12 |
|
A simple and rapid confirmatory assay for analyzing antibiot...
2007-01-01 [Rapid Commun. Mass Spectrom. 21(2) , 237-46, (2007)] |
|
Acid-catalyzed degradation of clarithromycin and erythromyci...
2000-02-10 [J. Med. Chem. 43(3) , 467-74, (2000)] |
|
An abiotic strategy for the enantioselective synthesis of er...
2003-07-21 [Angew. Chem. Int. Ed. Engl. 42(28) , 3278-81, (2003)] |
|
Design, synthesis, and evaluation of stable and taste-free e...
2005-06-02 [J. Med. Chem. 48(11) , 3878-84, (2005)] |
|
Chemical modification of erythromycins. IV. Synthesis and bi...
1990-05-01 [J. Antibiot. 43(5) , 544-9, (1990)] |