A simple and rapid confirmatory assay for analyzing antibiotic residues of the macrolide class and lincomycin in bovine milk and yoghurt: hot water extraction followed by liquid chromatography/tandem mass spectrometry.
Sara Bogialli, Antonio Di Corcia, Aldo Laganà, Veronica Mastrantoni, Manuel Sergi
文献索引:Rapid Commun. Mass Spectrom. 21(2) , 237-46, (2007)
全文:HTML全文
摘要
A rapid and simple sample preparation procedure for determining residues of antibiotics of the class of macrolides and lincomycin in whole milk and yoghurt by liquid chromatography/tandem mass spectrometry (LC/MS/MS) is presented. The method is based on the matrix solid-phase dispersion (MSPD) technique with hot water as extractant. After dispersing samples of milk and yoghurt on sand, target compounds were eluted from the MSPD column by passing through it 5 mL of water acidified with 30 mmol/L formic acid and heated at 70 degrees C. After pH adjustment and filtration, a volume of 200 microL of the aqueous extract was directly injected into the LC column. MS data acquisition was generally performed in the multiple reaction monitoring (MRM) mode, selecting two precursor ion to product ion transitions for each target compound. Hot water appeared to be an efficient extracting medium, since absolute recoveries of the analytes in milk and yoghurt were respectively 68-86% and 82-96%. The method proved to be robust as matrix effects, even though present, did not affect significantly the accuracy of the method, as evidenced by analyzing six different batches of both milk and yoghurt. Using roxithromycin (a macrolide antibiotic not used in veterinary medicine) as surrogate internal standard, the accuracy of the method at three different spike levels of the analytes in milk and yoghurt was 86-107% (RSDs not larger than 10%) and 97-117% (RSDs not larger than 13%), respectively. On the basis of a signal-to-noise ratio of 10, we estimated this method can quantify a few ppb of the analytes in milk and yoghurt. These concentrations are well below the tolerance levels of macrolides and lincomycin in milk set by both the European Union and the US Food and Drug Administration. On analyzing six yoghurt samples, we found evidence for the fact that one of the six samples was contaminated with erythromycin B.Copyright (c) 2006 John Wiley & Sons, Ltd.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
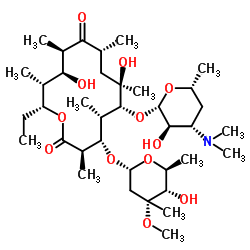 |
红霉素B
CAS:527-75-3 |
C37H67NO12 |
|
Acid-catalyzed degradation of clarithromycin and erythromyci...
2000-02-10 [J. Med. Chem. 43(3) , 467-74, (2000)] |
|
An abiotic strategy for the enantioselective synthesis of er...
2003-07-21 [Angew. Chem. Int. Ed. Engl. 42(28) , 3278-81, (2003)] |
|
Design, synthesis, and evaluation of stable and taste-free e...
2005-06-02 [J. Med. Chem. 48(11) , 3878-84, (2005)] |
|
An unexpected interaction between the modular polyketide syn...
2002-09-03 [Biochemistry 41(35) , 10827-33, (2002)] |
|
Chemical modification of erythromycins. IV. Synthesis and bi...
1990-05-01 [J. Antibiot. 43(5) , 544-9, (1990)] |