Organic Letters
2002-05-02
Ullmann diaryl ether synthesis: rate acceleration by 2,2,6,6-tetramethylheptane-3,5-dione.
Elizabeth Buck, Zhiguo Jake Song, David Tschaen, Peter G Dormer, R P Volante, Paul J Reider
文献索引:Org. Lett. 4(9) , 1623-6, (2002)
全文:HTML全文
摘要
[reaction: see text]. In the copper salt catalyzed ether formation from aryl bromides or iodides and phenols, 2,2,6,6-tetramethylheptane-3,5-dione (TMHD) was found to greatly accelerate the ordinarily difficult reaction, making it occur under more moderate temperatures and reaction times. A series of aryl halides and phenols were shown to form ethers in NMP as the solvent, cesium carbonate as the base, and CuCl and TMHD as the catalysts. The reaction was shown to tolerate electron-rich aryl bromides and electron-neutral phenols.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
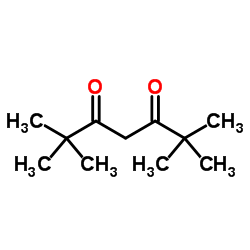 |
2,2,6,6-四甲基-3,5-庚二酮
CAS:1118-71-4 |
C11H20O2 |
相关文献:
更多...
|
26Mg as a probe in research on the role of magnesium in nutr...
1982-10-01 [Fed. Proc. 41(10) , 2709-13, (1982)] |
|
Ultrafine 239PuO2 aerosol generation, characterization and s...
1980-09-01 [Health Phys. 39(3) , 505-19, (1980)] |
|
Wavelength dependent photofragmentation patterns of tris(2,2...
2006-06-29 [J. Phys. Chem. A 110(25) , 7751-4, (2006)] |
|
[Tetrahedron 48 , 6909, (1992)] |
|
On the role of the bridging dicyanamidobenzene ligand in a n...
2006-11-13 [Inorg. Chem. 45(23) , 9332-45, (2006)] |