Expression in Escherichia coli, refolding, and purification of human procathepsin K, an osteoclast-specific protease.
K J D'alessio, M S McQueney, K A Brun, M J Orsini, C M Debouck
文献索引:Protein Expr. Purif. 15(2) , 213-20, (1999)
全文:HTML全文
摘要
We have constructed and optimized a high yielding Escherichia coli expression system to produce glycosylation-free human procathepsin K and have developed conditions for refolding this enzyme. Recombinant human procathepsin K (EC 3.4.22.38) was expressed in E. coli, refolded from inclusion bodies, and further purified by Superdex 75 size-exclusion chromatography. Purified procathepsin K had a [MH]+ of 35,063 Da which is in agreement with the predicted mass of the construct. Amino-terminal sequence analysis matched the predicted sequence with no secondary sequence detected. Purified procathepsin K activated under autocatalytic conditions to a final specific activity of 23 micromol 7-amido-4-methylcoumarin liberated/min/mg of enzyme using the fluorescent peptide substrate benzyloxycarbonyl-phenylalanine-arginine-7-amido-4-methylcoumarin. This expression and refolding procedure yielded 50 mg of purified, glycosylation-free human procathepsin K from 1 liter of E. coli cell culture and enabled the determination of the structure of human procathepsin K at 2.6 A resolution.Copyright 1999 Academic Press.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
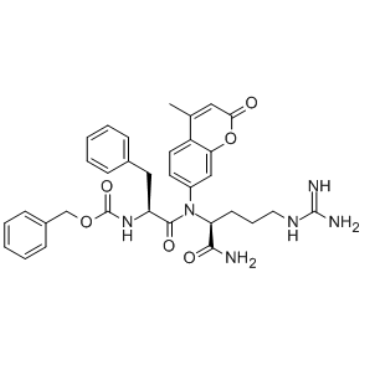 |
Z-苯丙胺酰-精氨酸-7-氨基-4-甲基香豆素盐酸盐
CAS:65147-22-0 |
C33H36N6O6 |
|
Assay of coagulation proteases using peptide chromogenic and...
1981-01-01 [Meth. Enzymol. 80 , 341, (1981)] |
|
Residue-specific annotation of disorder-to-order transition ...
2013-01-01 [PLoS ONE 8(1) , e54187, (2013)] |
|
Production of anti-peptide antibodies against trypanopain-Tb...
1997-06-01 [Immunopharmacology 36(2-3) , 295-303, (1997)] |
|
Modulation of the catalytic activity of cruzipain, the major...
2001-06-01 [Eur. J. Biochem. 268(11) , 3253-8, (2001)] |
|
Delineating functionally important regions and residues in t...
1996-09-09 [FEBS Lett. 393(1) , 24-6, (1996)] |