Modulation of the catalytic activity of cruzipain, the major cysteine proteinase from Trypanosoma cruzi, by temperature and pH.
L Salvati, M Mattu, F Polticelli, F Tiberi, L Gradoni, G Venturini, M Bolognesi, P Ascenzi
文献索引:Eur. J. Biochem. 268(11) , 3253-8, (2001)
全文:HTML全文
摘要
Cysteine proteinases are relevant to several aspects of the parasite life cycle and of parasite-host relationships. Here, a quantitative investigation of the effect of temperature and pH on the total substrate inhibition of cruzipain, the major papain-like cysteine proteinase from Trypanosoma cruzi, is reported. Values of the apparent catalytic and inhibition parameters Km, Vmax, Vmax/Km, and K(i) for the cruzipain-catalysed hydrolysis of N-alpha-benzyloxycarbonyl-L-phenylalanyl-L-arginine-(7-amino-4-methylcoumarin) (Z-Phe-Arg-AMC) and azocasein were determined between 10.0 degrees C and 40.0 degrees C and between pH 4.5 and 8.5. Values of Km were independent of temperature and pH, whereas values of Vmax, Vmax/Km, and K(i) were temperature-dependent and pH-dependent. Over the whole pH range explored, values of logVmax, log(Vmax/Km), and logK(i) increased linearly with respect to T(-1). Values of Vmax and Vmax/Km were affected by the acid-base equilibrium of one temperature-independent ionizing group (i.e. pK(unl)' = pK(lig)' = 5.7 +/- 0.1, at 25.0 degrees C). Moreover, values of K(i) were affected by the alkaline pK shift of one ionizing group of active cruzipain (from pK(unl)" = 5.7 +/- 0.1 to pK(lig)" = 6.1 +/- 0.1, at 25.0 degrees C) upon Z-Phe-Arg-AMC binding. Values of logK(unl)', logK(lig)', and logK(lig)" were temperature-independent. Conversely, values of logK(unl)" were linearly dependent on T(-1). As a whole, total substrate inhibition of cruzipain decreased with increasing temperature and pH. These data suggest that both synthetic and protein substrates can bind to the unique active centre of cruzipain either productively or following a binding mode which results in enzyme inhibition. However, allosteric effect(s) cannot be excluded.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
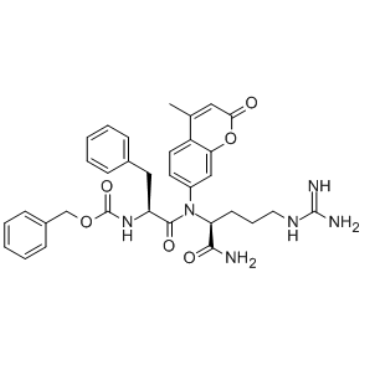 |
Z-苯丙胺酰-精氨酸-7-氨基-4-甲基香豆素盐酸盐
CAS:65147-22-0 |
C33H36N6O6 |
|
Assay of coagulation proteases using peptide chromogenic and...
1981-01-01 [Meth. Enzymol. 80 , 341, (1981)] |
|
Residue-specific annotation of disorder-to-order transition ...
2013-01-01 [PLoS ONE 8(1) , e54187, (2013)] |
|
Production of anti-peptide antibodies against trypanopain-Tb...
1997-06-01 [Immunopharmacology 36(2-3) , 295-303, (1997)] |
|
Delineating functionally important regions and residues in t...
1996-09-09 [FEBS Lett. 393(1) , 24-6, (1996)] |
|
Trypanosomatid cysteine protease activity may be enhanced by...
1995-01-15 [Biochem. J. 305 ( Pt 2) , 549-56, (1995)] |