Biotechnology and Applied Biochemistry
1993-02-01
Immobilization of beta-glucosidase from Penicillium funiculosum on nylon powder.
J Aguado, M D Romero, L Rodríguez
文献索引:Biotechnol. Appl. Biochem. 17 ( Pt 1) , 49-55, (1993)
全文:HTML全文
摘要
beta-Glucosidase from Penicillium funiculosum was immobilized on nylon powder previously activated with triethyloxonium tetrafluoroborate, 1,2-diaminoethane and glutaraldehyde. The activation of the nylon powder and the immobilization processes were studied and optimized for the enzyme and the matrix. A high activity retention (67%) was obtained using the activation and immobilization conditions finally selected.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
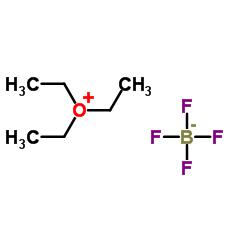 |
三乙基氧鎓四氟硼酸盐
CAS:368-39-8 |
C6H15BF4O |
相关文献:
更多...
|
Determination of thiocyanate in saliva by headspace gas chro...
2015-06-26 [J. Chromatogr. A. 1400 , 124-30, (2015)] |
|
Method for activation and recycling of trityl resins.
2012-08-17 [J. Org. Chem. 77(16) , 7071-5, (2012)] |
|
Negative chemical ionization GC/MS determination of nitrite ...
2012-03-06 [Anal. Chem. 84(5) , 2592-6, (2012)] |
|
The specificity and kinetic properties of trypsin ethylated ...
1985-01-28 [FEBS Lett. 180(2) , 239-42, (1985)] |
|
High yielding synthesis of N-ethyl dehydroamino acids.
2012-10-01 [Amino Acids 43(4) , 1643-52, (2012)] |