Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer.
Gerhardt Attard, Alison H M Reid, Roger A'Hern, Christopher Parker, Nikhil Babu Oommen, Elizabeth Folkerd, Christina Messiou, L Rhoda Molife, Gal Maier, Emilda Thompson, David Olmos, Rajesh Sinha, Gloria Lee, Mitch Dowsett, Stan B Kaye, David Dearnaley, Thian Kheoh, Arturo Molina, Johann S de Bono
文献索引:J. Clin. Oncol. 27(23) , 3742-8, (2009)
全文:HTML全文
摘要
It has been postulated that castration-resistant prostate cancer (CRPC) commonly remains hormone dependent. Abiraterone acetate is a potent, selective, and orally available inhibitor of CYP17, the key enzyme in androgen and estrogen biosynthesis.This was a phase I/II study of abiraterone acetate in castrate, chemotherapy-naive CRPC patients (n = 54) with phase II expansion at 1,000 mg (n = 42) using a two-stage design to reject the null hypothesis if more than seven patients had a prostate-specific antigen (PSA) decline of > or = 50% (null hypothesis = 0.1; alternative hypothesis = 0.3; alpha = .05; beta = .14). Computed tomography scans every 12 weeks and circulating tumor cell (CTC) enumeration were performed. Prospective reversal of resistance at progression by adding dexamethasone 0.5 mg/d to suppress adrenocorticotropic hormone and upstream steroids was pursued.A decline in PSA of > or = 50% was observed in 28 (67%) of 42 phase II patients, and declines of > or = 90% were observed in eight (19%) of 42 patients. Independent radiologic evaluation reported partial responses (Response Evaluation Criteria in Solid Tumors) in nine (37.5%) of 24 phase II patients with measurable disease. Decreases in CTC counts were also documented. The median time to PSA progression (TTPP) on abiraterone acetate alone for all phase II patients was 225 days (95% CI, 162 to 287 days). Exploratory analyses were performed on all 54 phase I/II patients; the addition of dexamethasone at disease progression reversed resistance in 33% of patients regardless of prior treatment with dexamethasone, and pretreatment serum androgen and estradiol levels were associated with a probability of > or = 50% PSA decline and TTPP on abiraterone acetate and dexamethasone.CYP17 blockade by abiraterone acetate results in declines in PSA and CTC counts and radiologic responses, confirming that CRPC commonly remains hormone driven.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
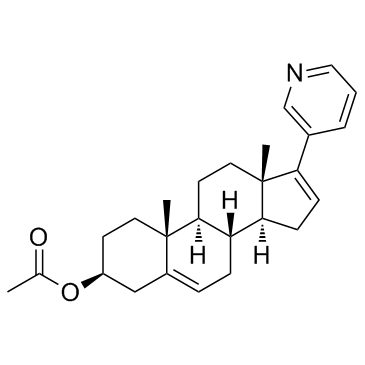 |
乙酸阿比特龙酯
CAS:154229-18-2 |
C26H33NO2 |
|
Phase I clinical trial of the CYP17 inhibitor abiraterone ac...
2010-03-20 [J. Clin. Oncol. 28(9) , 1481-8, (2010)] |
|
Significant and sustained antitumor activity in post-docetax...
2010-03-20 [J. Clin. Oncol. 28(9) , 1489-95, (2010)] |
|
Phase II multicenter study of abiraterone acetate plus predn...
2010-03-20 [J. Clin. Oncol. 28(9) , 1496-501, (2010)] |
|
Treating Patients with Metastatic Castration Resistant Prost...
2015-12-01 [J. Urol. 194(6) , 1537-47, (2015)] |
|
Antitumor activity with CYP17 blockade indicates that castra...
2009-06-15 [Cancer Res. 69(12) , 4937-40, (2009)] |