| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
7-苄氧基-3H-吩恶嗪-3-酮
CAS:87687-02-3 |
|
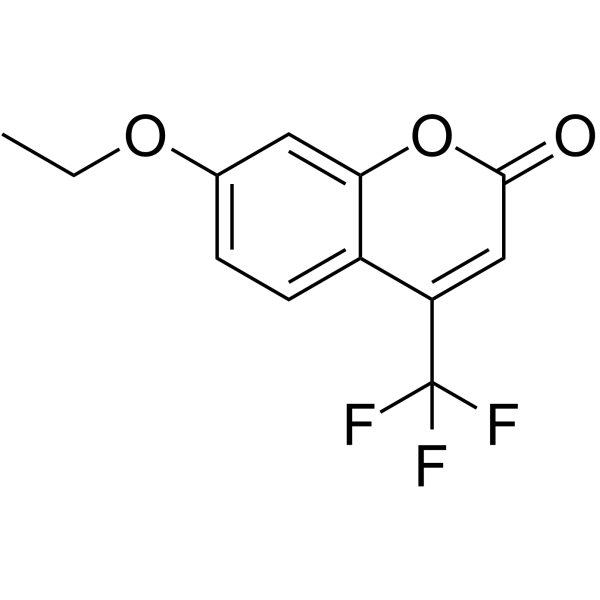 |
7-乙氧基-4-(三氟甲基)香豆素
CAS:115453-82-2 |