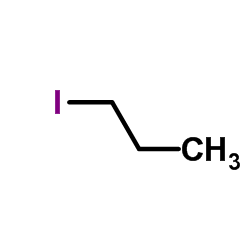
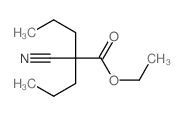
|
~64% |
|
~76% |
|
~% |
|
~% |
|
~84% |
|
~% |
|
~87% |
|
~93% |

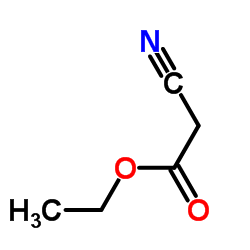
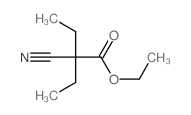
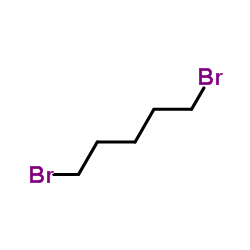
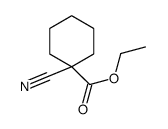

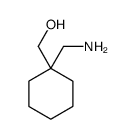
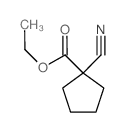
![[1-(氨基甲基)环戊基]甲醇结构式](https://image.chemsrc.com/caspic/304/2239-31-8.png)