| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
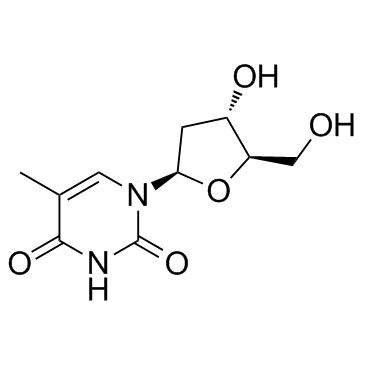 |
beta-胸苷
CAS:50-89-5 |
|
 |
5-溴-2'-脱氧尿苷
CAS:59-14-3 |
|
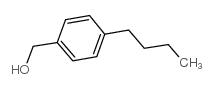 |
对丁基苯甲醇
CAS:60834-63-1 |