Installation of the pyrrolyl-2-carboxyl pharmacophore by CouN1 and CouN7 in the late biosynthetic steps of the aminocoumarin antibiotics clorobiocin and coumermycin A1.
Sylvie Garneau-Tsodikova, Anthony Stapon, Daniel Kahne, Christopher T Walsh
文献索引:Biochemistry 45(28) , 8568-78, (2006)
全文:HTML全文
摘要
The 5-methyl-2-pyrrolylcarbonyl moiety of the aminocoumarin antibiotics clorobiocin and coumermycin A1 is the key pharmacophore for targeting the ATP-binding site of GyrB for inhibition of the bacterial type-II topoisomerase DNA gyrase. During the late stage of clorobiocin and coumermycin A1 biosynthesis, the pyrrolyl-2-carboxyl group is transferred from the peptidyl carrier proteins Clo/CouN1 to the 3'-hydroxyl of the 4-methoxy-L-noviosyl scaffold by the action of the acyltransferases Clo/CouN7. CouN1 and CouN7 have now been heterologously expressed and purified from Escherichia coli. The apo form of CouN1 is converted to the acyl-holo form by loading with pyrrolyl-2-carboxyl-S-pantetheinyl moieties from synthetic pyrrolyl- and 5-methylpyrrolyl-CoAs by the action of the phosphopantetheinyl transferase Sfp. CouN7 acts as an acyltransferase, moving the pyrrole acyl moieties from CouN1 to the noviose sugar of descarbamoylnovobiocin. When the 5-methylpyrrolyl-2-carboxyl-thioester of CouN1 is the cosubstrate, the in vitro product differs from clorobiocin only in a CH3 for Cl group change on the coumarin ring. Double transfer of this acyl moiety by CouN7 to the penultimate intermediate in coumermycin A1 assembly completes that antibiotic biosynthetic pathway.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
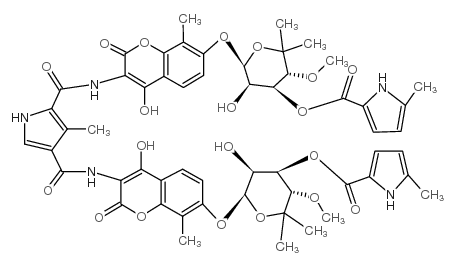 |
Notomycin A1
CAS:4434-05-3 |
C55H59N5O20 |
|
Multiplexing interactions to control antibiotic release from...
2011-11-10 [Macromol. Biosci. 11(11) , 1544-52, (2011)] |
|
Aminocoumarins inhibit osteoclast differentiation and bone r...
2013-02-01 [Biochem. Pharmacol. 85(3) , 417-25, (2013)] |
|
Modulation of chaperone function and cochaperone interaction...
2006-03-17 [J. Biol. Chem. 281 , 7161-7171, (2006)] |
|
Synthesis and characterization of PEG-based drug-responsive ...
2012-08-14 [Macromol. Rapid Commun. 33(15) , 1280-5, (2012)] |
|
Covalent CouN7 enzyme intermediate for acyl group shuttling ...
2007-06-01 [Chem. Biol. 14(6) , 679-90, (2007)] |