CouO and NovO: C-methyltransferases for tailoring the aminocoumarin scaffold in coumermycin and novobiocin antibiotic biosynthesis.
Michelle Pacholec, Junhua Tao, Christopher T Walsh
文献索引:Biochemistry 44(45) , 14969-76, (2005)
全文:HTML全文
摘要
During the biosynthesis of the streptomycete aminocoumarin antibiotics novobiocin and the dimeric coumermycin A(1), the bicyclic coumarin scaffold is C-methylated adjacent to the phenolic oxygen. The SAM-dependent C-methyltransferases NovO and CouO have been heterologously expressed and purified from Escherichia coli and shown to act after the aminocoumarin ring has been constructed by prior action of Nov/CouHIJK. Neither C-methyltransferase works on the tyrosyl-derived S-pantetheinyl intermediates tethered to NovH or on the subsequently released free aminocoumarin. NovL ligates the aminocoumarin to prenylhydroxybenzoate to yield novobiocic acid, which is the substrate for NovO before it is O-glycosylated by NovM. In coumermycin assembly, the corresponding ligase CouL makes the bis-amide by tandem ligation of two aminocoumarins to a dicarboxypyrrole. CouO works on both the mono- and bis-amides for mono- and di-C-methylation adjacent to the phenolic hydroxyl before it is glycosylated by CouM. Thus, the specific timing of C-methylation in the aminocoumarin antibiotic pathways is established.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
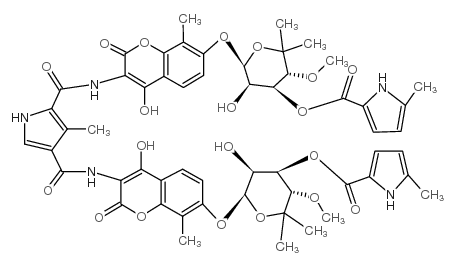 |
Notomycin A1
CAS:4434-05-3 |
C55H59N5O20 |
|
Multiplexing interactions to control antibiotic release from...
2011-11-10 [Macromol. Biosci. 11(11) , 1544-52, (2011)] |
|
Aminocoumarins inhibit osteoclast differentiation and bone r...
2013-02-01 [Biochem. Pharmacol. 85(3) , 417-25, (2013)] |
|
Modulation of chaperone function and cochaperone interaction...
2006-03-17 [J. Biol. Chem. 281 , 7161-7171, (2006)] |
|
Synthesis and characterization of PEG-based drug-responsive ...
2012-08-14 [Macromol. Rapid Commun. 33(15) , 1280-5, (2012)] |
|
Covalent CouN7 enzyme intermediate for acyl group shuttling ...
2007-06-01 [Chem. Biol. 14(6) , 679-90, (2007)] |