| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
D-(+)-纤维四糖
CAS:38819-01-1 |
|
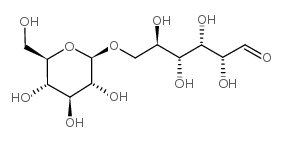 |
β-龙胆二糖
CAS:554-91-6 |
|
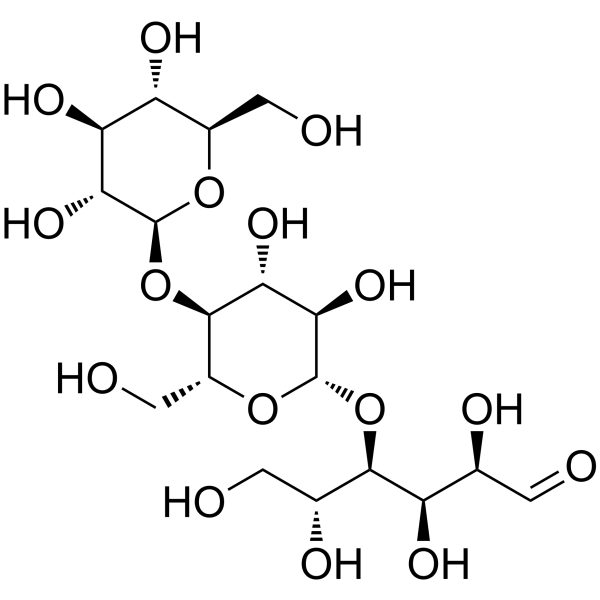 |
D-(+)-纤维三糖
CAS:33404-34-1 |
|
 |
麦芽糖
CAS:69-79-4 |