| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
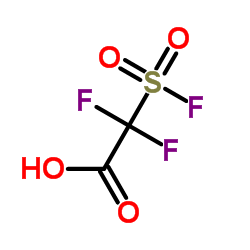 |
2,2-二氟-2-(氟磺酰基)乙酸
CAS:1717-59-5 |
|
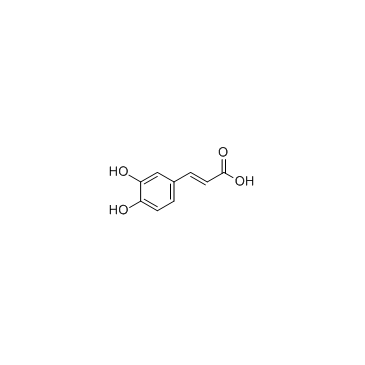 |
咖啡酸
CAS:331-39-5 |
|
 |
布洛芬
CAS:15687-27-1 |