Regioselective bond cleavage in the dissociative electron transfer to benzyl thiocyanates.
Abdelaziz Houmam, Emad M Hamed, Philippe Hapiot, John M Motto, Adrian L Schwan
文献索引:J. Am. Chem. Soc. 125(42) , 12676-7, (2003)
全文:HTML全文
摘要
The electrochemical reduction of benzyl thiocyanate and p-nitrobenzyl thiocyanate was investigated in acetonitrile at an inert electrode. These two compounds reveal a change in the reductive cleavage mechanism, and more interestingly, they show a clear-cut example of a regioselective bond dissociation. Both phenomena may be understood on the basis of the dissociative ET theory and its extension to the formation/dissociation reactions of radical ions. While the effect of the standard oxidation potential of the leaving group seems to be predominant in understanding the change in the ET mechanism by changing the driving force, the regioselective cleavage is dictated by changes in the intrinsic barrier related to the nature of the substituent on the aryl moiety.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
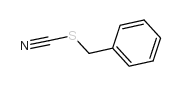 |
硫氰酸苯甲酯
CAS:3012-37-1 |
C8H7NS |
|
In vitro metabolic conversion of the organic breakdown produ...
2015-08-30 [J. Sci. Food Agric. 95 , 2244-51, (2015)] |
|
Subcellular localization of enzymes in Streptomyces aureofac...
1987-01-01 [Folia Microbiol. (Praha) 32(5) , 402-10, (1987)] |
|
Protein profiles of Streptomyces aureofaciens producing tetr...
1995-08-01 [Curr. Microbiol. 31(2) , 84-91, (1995)] |
|
Effect of some benzyl thiocyanate analogs on tetracycline pr...
1987-09-01 [J. Antibiot. 40(9) , 1341-3, (1987)] |
|
Effects of dietary compounds on alpha-hydroxylation of N-nit...
1984-07-01 [Cancer Res. 44(7) , 2924-2928, (1984)] |