Effects of dietary compounds on alpha-hydroxylation of N-nitrosopyrrolidine and N'-nitrosonornicotine in rat target tissues.
F L Chung, A Juchatz, J Vitarius, S S Hecht
文献索引:Cancer Res. 44(7) , 2924-2928, (1984)
全文:HTML全文
摘要
Male F344 rats were pretreated with various dietary compounds, and the effects of pretreatment on the in vitro alpha-hydroxylation of N-nitrosopyrrolidine or N'-nitrosonornicotine were determined in assays with liver microsomes or cultured esophagus, respectively. Dietary compounds included phenols, cinnamic acids, coumarins, indoles, and isothiocyanates. Pretreatments were carried out either by administering the compound by gavage 2 hr prior to sacrifice (acute protocol) or by adding the compound to the diet for 2 weeks (chronic protocol). Acute pretreatment with benzyl isothiocyanate, allyl isothiocyanate, phenethyl isothiocyanate, phenyl isocyanate, and benzyl thiocyanate but not sodium thiocyanate inhibited formation of alpha-hydroxylation products of both nitrosamines. When the chronic pretreatment protocol was used, only phenyl isothiocyanate and sodium thiocyanate inhibited formation of alpha-hydroxylation products of both nitrosamines. Pretreatments with butylated hydroxyanisole, p-methoxyphenol, or N-acetylcysteine had little, if any, effect on the alpha-hydroxylation of N-nitrosopyrrolidine or N'-nitrosonornicotine. Chronic pretreatment with p-hydroxycinnamic acid, 4-hydroxy-3- methoxycinnamic acid, coumarin, umbelliferone, limetine , indole, indole-3-carbinol, indole-3-acetonitrile, and L-tryptophan induced activity for the alpha-hydroxylation of N-nitrosopyrrolidine. The results of this study indicate that isothiocyanates are possible candidates for further study as potential inhibitors of carcinogenesis by N-nitrosopyrrolidine and N'-nitrosonornicotine.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
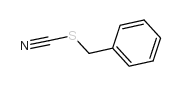 |
硫氰酸苯甲酯
CAS:3012-37-1 |
C8H7NS |
|
In vitro metabolic conversion of the organic breakdown produ...
2015-08-30 [J. Sci. Food Agric. 95 , 2244-51, (2015)] |
|
Subcellular localization of enzymes in Streptomyces aureofac...
1987-01-01 [Folia Microbiol. (Praha) 32(5) , 402-10, (1987)] |
|
Protein profiles of Streptomyces aureofaciens producing tetr...
1995-08-01 [Curr. Microbiol. 31(2) , 84-91, (1995)] |
|
Effect of some benzyl thiocyanate analogs on tetracycline pr...
1987-09-01 [J. Antibiot. 40(9) , 1341-3, (1987)] |
|
Regioselective bond cleavage in the dissociative electron tr...
2003-10-22 [J. Am. Chem. Soc. 125(42) , 12676-7, (2003)] |