JAMA Pediatrics
2014-04-01
Disease mechanisms and clonidine treatment in adolescent chronic fatigue syndrome: a combined cross-sectional and randomized clinical trial.
Dag Sulheim, Even Fagermoen, Anette Winger, Anders Mikal Andersen, Kristin Godang, Fredrik Müller, Peter C Rowe, J Philip Saul, Eva Skovlund, Merete Glenne Øie, Vegard Bruun Wyller
文献索引:JAMA Pediatr. 168(4) , 351-60, (2014)
全文:HTML全文
摘要
Chronic fatigue syndrome (CFS) is a disabling condition with unknown disease mechanisms and few treatment options.To explore the pathophysiology of CFS and assess clonidine hydrochloride pharmacotherapy in adolescents with CFS by using a hypothesis that patients with CFS have enhanced sympathetic activity and that sympatho-inhibition by clonidine would improve symptoms and function.Participants were enrolled from a single referral center recruiting nationwide in Norway. A referred sample of 176 adolescents with CFS was assessed for eligibility; 120 were included (34 males and 86 females; mean age, 15.4 years). A volunteer sample of 68 healthy adolescents serving as controls was included (22 males and 46 females; mean age, 15.1 years). The CSF patients and healthy controls were assessed cross-sectionally at baseline. Thereafter, patients with CFS were randomized 1:1 to treatment with low-dose clonidine or placebo for 9 weeks and monitored for 30 weeks; double-blinding was provided. Data were collected from March 2010 until October 2012 as part of the Norwegian Study of Chronic Fatigue Syndrome in Adolescents: Pathophysiology and Intervention Trial.Clonidine hydrochloride capsules (25 µg or 50 µg twice daily for body weight <35 kg or >35 kg, respectively) vs placebo capsules for 9 weeks.Number of steps per day.At baseline, patients with CFS had a lower number of steps per day (P < .001), digit span backward score (P = .002), and urinary cortisol to creatinine ratio (P = .001), and a higher fatigue score (P < .001), heart rate responsiveness (P = .02), plasma norepinephrine level (P < .001), and serum C-reactive protein concentration (P = .04) compared with healthy controls. There were no significant differences regarding blood microbiology evaluation. During intervention, the clonidine group had a lower number of steps per day (mean difference, -637 steps; P = .07), lower plasma norepinephrine level (mean difference, -42 pg/mL; P = .01), and lower serum C-reactive protein concentration (mean ratio, 0.69; P = .02) compared with the CFS placebo group.Adolescent CFS is associated with enhanced sympathetic nervous activity, low-grade systemic inflammation, attenuated hypothalamus-pituitary-adrenal axis function, cognitive impairment, and large activity reduction, but not with common microorganisms. Low-dose clonidine attenuates sympathetic outflow and systemic inflammation in CFS but has a concomitant negative effect on physical activity; thus, sympathetic and inflammatory enhancement may be compensatory mechanisms. Low-dose clonidine is not clinically useful in CFS.clinicaltrials.gov Identifier: NCT01040429.
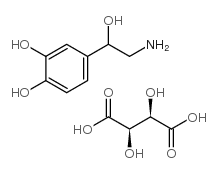
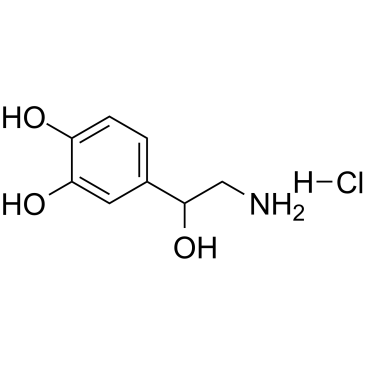
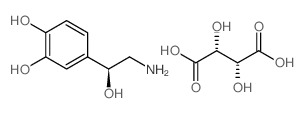
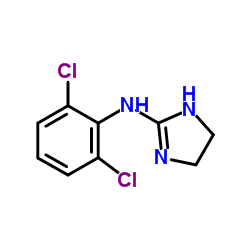
相关化合物