| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
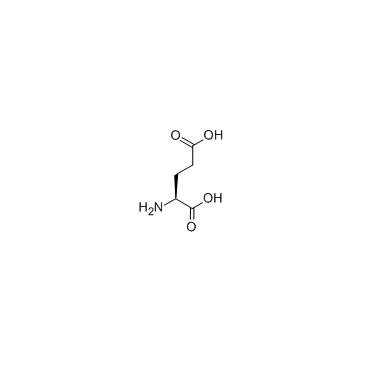 |
L-谷氨酸
CAS:56-86-0 |
|
 |
L-O-磷酸丝氨酸
CAS:407-41-0 |
|
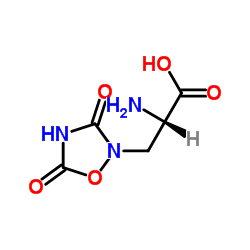 |
异喹啉酸(mM/ml)
CAS:52809-07-1 |