Structural characterization of the papaya cysteine proteinases at low pH.
Joëlle Huet, Yvan Looze, Kristin Bartik, Vincent Raussens, René Wintjens, Paule Boussard
文献索引:Biochem. Biophys. Res. Commun. 341(2) , 620-6, (2006)
全文:HTML全文
摘要
Current control of gastrointestinal nematodes relies primarily on the use of synthetic drugs and encounters serious problems of resistance. Oral administration of plant cysteine proteinases, known to be capable of damaging nematode cuticles, has recently been recommended to overcome these problems. This prompted us to examine if plant cysteine proteinases like the four papaya proteinases papain, caricain, chymopapain, and glycine endopeptidase that have been investigated here can survive acidic pH conditions and pepsin degradation. The four papaya proteinases have been found to undergo, at low pH, a conformational transition that instantaneously converts their native forms into molten globules that are quite unstable and rapidly degraded by pepsin. As shown by activity measurements, the denatured state of these proteinases which finally results from acid treatment is completely irreversible. It is concluded that cysteine proteinases from plant origin may require to be protected against both acid denaturation and proteolysis to be effective in the gut after oral administration.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
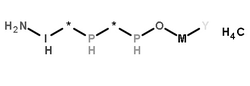 |
糜木瓜酶
CAS:9001-09-6 |
H5M*2NOP2YI.CH4 |
|
Purification and characterization of extracellular cysteine ...
2006-02-01 [J. Biosci. Bioeng. 101(2) , 166-71, (2006)] |
|
Surface Plasmon Resonance Imaging biosensor for cystatin det...
2012-01-01 [Folia Histochem. Cytobiol. 50(1) , 130-6, (2012)] |
|
Papaya latex enzymes capable of detoxification of gliadin.
2010-01-01 [Amino Acids 38(1) , 155-65, (2010)] |
|
Intradiscal therapies for discogenic pain.
2006-06-01 [Semin. Musculoskelet. Radiol. 10(2) , 125-35, (2006)] |
|
Study of blood metabolism and urinary excretion of chymopapa...
2005-01-01 [J. Orthop. Sci. 10(2) , 206-13, (2005)] |