| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
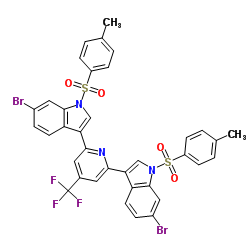 |
硫氧还蛋白还原酶
CAS:9074-14-0 |
|
 |
硫氧还蛋白 来源于大肠杆菌
CAS:52500-60-4 |