| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
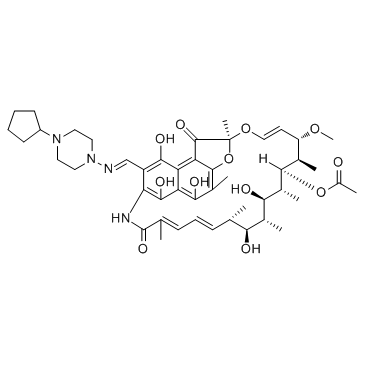 |
利福喷丁
CAS:61379-65-5 |
|
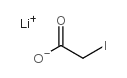 |
碘乙酸锂
CAS:65749-30-6 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
利福喷丁
CAS:61379-65-5 |
|
 |
碘乙酸锂
CAS:65749-30-6 |