Evaluation of new migrastatin and dorrigocin congeners unveils cell migration inhibitors with dramatically improved potency.
Jianhua Ju, Scott R Rajski, Si-Kyu Lim, Jeong-Woo Seo, Noël R Peters, F Michael Hoffmann, Ben Shen
文献索引:Bioorg. Med. Chem. Lett. 18(22) , 5951-4, (2008)
全文:HTML全文
摘要
Lactimidomycin (LTM, 1), iso-migrastatin (iso-MGS, 2) and migrastatin (MGS, 3) are macrolide antitumor antibiotics differing in macrolide ring size but all bearing a glutarimide side chain. To further develop these natural products and related analogs as drug candidates we have produced and evaluated the biological activities of a small library of iso-MGS and LTM-derived agents; congeners evaluated bear either the MGS scaffold or related acyclic (dorrigocin) scaffolds. Scratch wound-healing (SWH) assays with 4T1 mouse and MDA-MB-231 human mammary tumor cell lines, respectively, reveal structural elements crucial to inhibition of cell migration by these compounds. Moreover, two substances, 14 and 17, with activity far superior to that of MGS are unveiled by SWH assays.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
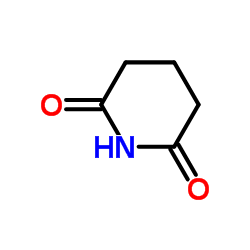 |
戊二酰亚胺
CAS:1121-89-7 |
C5H7NO2 |
|
Comparative characterization of the lactimidomycin and iso-m...
2014-12-16 [Biochemistry 53(49) , 7854-65, (2014)] |
|
Iso-migrastatin congeners from Streptomyces platensis and ge...
2005-08-31 [J. Am. Chem. Soc. 127(34) , 11930-1, (2005)] |
|
Enantiomerization mechanism of thalidomide and the role of w...
2012-11-05 [Chemistry 18(45) , 14305-13, (2012)] |
|
Inhibition of macrophage activation and suppression of graft...
2011-09-01 [Inflamm. Res. 60(9) , 879-88, (2011)] |
|
Detailed studies on the enantioselective synthesis and HPLC ...
2005-11-01 [Chirality 17(9) , 595-9, (2005)] |