Iso-migrastatin congeners from Streptomyces platensis and generation of a glutarimide polyketide library featuring the dorrigocin, lactimidomycin, migrastatin, and NK30424 scaffolds.
Jianhua Ju, Si-Kyu Lim, Hui Jiang, Jeong-Woo Seo, Ben Shen
文献索引:J. Am. Chem. Soc. 127(34) , 11930-1, (2005)
全文:HTML全文
摘要
Iso-Migrastatin (10) has been shown to be the main natural product of Streptomyces platensis, which undergoes a facile, H2O-mediated rearrangement into dorrigocin A (2), 13-epi-dorrigocin A (11), dorrigocin B (3), and migrastatin (1). Eight new congeners (12-19) of 10 were characterized. They can undergo the same H2O-mediated rearrangement into the corresponding 1, 2, 3, and 11 analogues (20-43) or 1,4-Michael addition with cysteine to afford the corresponding analogues (44-51) of NK30424 A and B (5, 6). This study generated a 47-member library of glutarimide polyketides, setting the stage to investigate the SAR for this family of natural products. These results also established the absolute stereochemistry of 5 and 6 and shed new light into the post-polyketide synthase steps for 10 biosynthesis.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
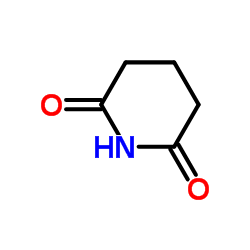 |
戊二酰亚胺
CAS:1121-89-7 |
C5H7NO2 |
|
Comparative characterization of the lactimidomycin and iso-m...
2014-12-16 [Biochemistry 53(49) , 7854-65, (2014)] |
|
Enantiomerization mechanism of thalidomide and the role of w...
2012-11-05 [Chemistry 18(45) , 14305-13, (2012)] |
|
Evaluation of new migrastatin and dorrigocin congeners unvei...
2008-11-15 [Bioorg. Med. Chem. Lett. 18(22) , 5951-4, (2008)] |
|
Inhibition of macrophage activation and suppression of graft...
2011-09-01 [Inflamm. Res. 60(9) , 879-88, (2011)] |
|
Detailed studies on the enantioselective synthesis and HPLC ...
2005-11-01 [Chirality 17(9) , 595-9, (2005)] |