Metabolism of benzamidoxime (N-hydroxyamidine) in human hepatocytes and role of UDP-glucuronosyltransferases.
A K Fröhlich, U Girreser, B Clement
文献索引:Xenobiotica 35(1) , 17-25, (2005)
全文:HTML全文
摘要
N-Hydroxyamidines (amidoximes) can act as pro-drugs of amidines (e.g. ximelagatran, a novel direct thrombin inhibitor). This known pro-drug principle is based on the N-reduction of an oral bioavailable amidoxime to its active form. Previous study of the metabolism of the model substrate benzamidoxime by pig hepatocytes demonstrated the formation of benzamidoxime-O-glucuronide in addition to the well-established N-reduction. The objective of the present work was to investigate the glucuronidation of benzamidoxime by using cultivated cryopreserved human hepatocytes. Furthermore, the involvement of human UDP-glucuronosyltransferases (UGTs) was examined by incubating benzamidoxime in the presence of eight human hepatic recombinant UGT enzymes. Metabolites were analysed by liquid chromatography/mass spectrometry using electrospray ionization and compared with authentic synthetic compounds. For the first time, the O-glucuronidation of benzamidoxime was demonstrated in cultures of human hepatocytes. UGT1A9 is the most efficient enzyme conjugating benzamidoxime, whereas the conversion activities of UGT1A1 and UGT1A3 were 60-fold lower. Human hepatocytes form two non-mutagenic compounds: benzamidine, as the predominating metabolite, and benzamidoxime-O-glucuronide to a lesser extent. N-oxidation of benzamidine was not detected.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
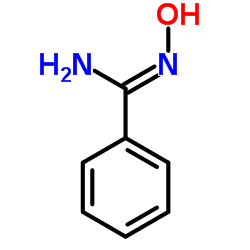 |
苄胺肟
CAS:613-92-3 |
C7H8N2O |
|
A novel and specific fluorescence reaction for uracil.
2010-08-03 [Anal. Chim. Acta 674(2) , 234-8, (2010)] |
|
Characteristics of the microsomal N-hydroxylation of benzami...
1987-06-01 [Xenobiotica 17(6) , 659-67, (1987)] |
|
Biotransformation of benzamidine and benzamidoxime in vivo.
1993-10-01 [Arch. Pharm. (Weinheim) 326(10) , 807-12, (1993)] |
|
Genotoxic activities of benzamidine and its N-hydroxylated m...
1988-01-01 [J. Cancer Res. Clin. Oncol. 114(4) , 363-8, (1988)] |
|
[Biotransformation of benzamidine and benzamidoxime by micro...
1989-07-01 [Arch. Pharm. (Weinheim) 322(7) , 431-5, (1989)] |