| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
2-溴苯乙酮
CAS:70-11-1 |
|
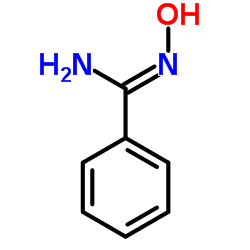 |
苄胺肟
CAS:613-92-3 |
|
 |
4-(三氟甲氧基)苄胺肟
CAS:56935-71-8 |
|
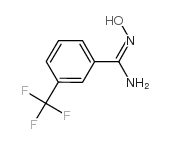 |
3-(三氟甲基)苄胺肟
CAS:40067-80-9 |