| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
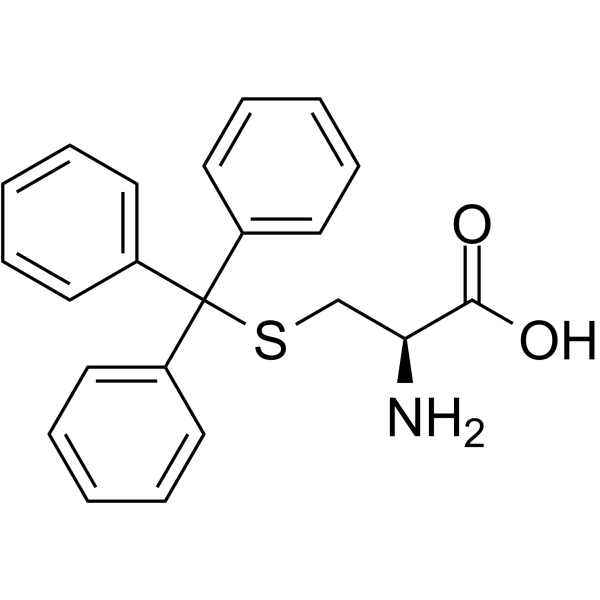 |
S-三苯甲基-L-半胱氨酸
CAS:2799-07-7 |
|
 |
胞内蛋白酶赖氨酸-C
CAS:72561-05-8 |