| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
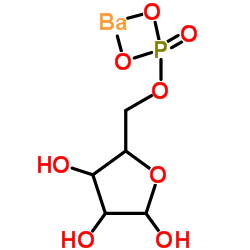 |
(2R,3R,4R)-2,3,4-三羟基-5-氧代戊基磷酸钡
CAS:15673-79-7 |
|
 |
Ribose-5-phosphate isomerase
CAS:9023-83-0 |