Defining the structural requirements for ribose 5-phosphate-binding and intersubunit cross-talk of the malarial pyridoxal 5-phosphate synthase.
Bianca Derrer, Volker Windeisen, Gabriela Guédez Rodríguez, Joerg Seidler, Martin Gengenbacher, Wolf D Lehmann, Karsten Rippe, Irmgard Sinning, Ivo Tews, Barbara Kappes
文献索引:FEBS Lett. 584(19) , 4169-74, (2010)
全文:HTML全文
摘要
Most organisms synthesise the B(6) vitamer pyridoxal 5-phosphate (PLP) via the glutamine amidotransferase PLP synthase, a large enzyme complex of 12 Pdx1 synthase subunits with up to 12 Pdx2 glutaminase subunits attached. Deletion analysis revealed that the C-terminus has four distinct functionalities: assembly of the Pdx1 monomers, binding of the pentose substrate (ribose 5-phosphate), formation of the reaction intermediate I(320), and finally PLP synthesis. Deletions of distinct C-terminal regions distinguish between these individual functions. PLP formation is the only function that is conferred to the enzyme by the C-terminus acting in trans, explaining the cooperative nature of the complex.Copyright © 2010 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
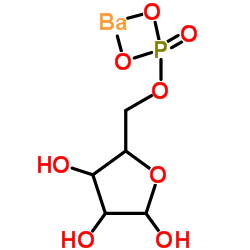 |
(2R,3R,4R)-2,3,4-三羟基-5-氧代戊基磷酸钡
CAS:15673-79-7 |
C5H9BaO8P |
|
Post-slaughter changes in ATP metabolites, reducing and phos...
2013-05-01 [Meat Science 94(1) , 55-62, (2013)] |
|
Effects of free Ca²⁺ on kinetic characteristics of holotrans...
[Protein J. 31(2) , 137-40, (2012)] |
|
Molecular differences between a mutase and a phosphatase: in...
2012-03-06 [Biochemistry 51(9) , 1964-75, (2012)] |
|
Ribose 5-phosphate glycation reduces cytochrome c respirator...
2011-12-27 [Biochemistry 50(51) , 11047-57, (2011)] |
|
Cooperative binding of substrates to transketolase from Sacc...
2009-07-01 [Biochemistry. (Mosc.) 74(7) , 789-92, (2009)] |