Open-closed conformational change revealed by the crystal structures of 3-keto-L-gulonate 6-phosphate decarboxylase from Streptococcus mutans.
Gui-Lan Li, Xiang Liu, Jie Nan, Erik Brostromer, Lan-Fen Li, Xiao-Dong Su
文献索引:Biochem. Biophys. Res. Commun. 381(3) , 429-33, (2009)
全文:HTML全文
摘要
The 3-keto-L-gulonate 6-phosphate decarboxylase (KGPDC) catalyses the decarboxylation of 3-keto-L-gulonate 6-phosphate to L-xylulose in the presence of magnesium ions. The enzyme is involved in L-ascorbate metabolism and plays an essential role in the pathway of glucuronate interconversion. Crystal structures of Streptococcus mutans KGPDC were determined in the absence and presence of the product analog D-ribulose 5-phosphate. We have observed an 8 A alphaB-helix movement and other structural rearrangements around the active site between the apo-structures and product analog bound structure. These drastic conformational changes upon ligand binding are the first observation of this kind for the KGPDC family. The flexibilities of both the alpha-helix lid and the side chains of Arg144 and Arg197 are associated with substrate binding and product releasing. The open-closed conformational changes of the active site, through the movements of the alpha-helix lid and the arginine residues are important for substrate binding and catalysis.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
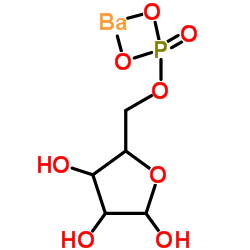 |
(2R,3R,4R)-2,3,4-三羟基-5-氧代戊基磷酸钡
CAS:15673-79-7 |
C5H9BaO8P |
|
Post-slaughter changes in ATP metabolites, reducing and phos...
2013-05-01 [Meat Science 94(1) , 55-62, (2013)] |
|
Effects of free Ca²⁺ on kinetic characteristics of holotrans...
[Protein J. 31(2) , 137-40, (2012)] |
|
Defining the structural requirements for ribose 5-phosphate-...
2010-10-08 [FEBS Lett. 584(19) , 4169-74, (2010)] |
|
Molecular differences between a mutase and a phosphatase: in...
2012-03-06 [Biochemistry 51(9) , 1964-75, (2012)] |
|
Ribose 5-phosphate glycation reduces cytochrome c respirator...
2011-12-27 [Biochemistry 50(51) , 11047-57, (2011)] |