Effect of protein side chain amide group on the hydrogen-bond equilibrium in nucleobases studied by infrared and 13C-NMR spectroscopy.
M Molina, P Carmona
文献索引:Biophys. Chem. 34(1) , 1-8, (1989)
全文:HTML全文
摘要
Infrared spectra of 1:1 hydrogen-bonded complexes formed by derivatives of adenine and model molecules bearing the protein side chain amide group have been measured in chloroform solution. From the temperature dependence of hydrogen-bond formation, thermodynamic data on these complexes are determined. On the basis of these data, it is shown that the complexes consist of cyclic heterodimers, those that use the adenine N(1)H bond being favoured. Similarly infrared and 13C-NMR spectroscopy reveals that uracil-amide cyclic heterodimers formed through the uracil 4-carbonyl group are predominant. All of these results predict that Watson-Crick hydrogen bonds in adenine-uracil base-pairs may be opened to some extent, as proved in this work. The possible biological importance of these observations is also discussed.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
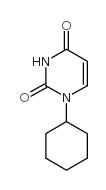 |
1-环己基尿嘧啶
CAS:712-43-6 |
C10H14N2O2 |
|
Solvent-dependent photophysics of 1-cyclohexyluracil: ultraf...
2006-09-21 [J. Phys. Chem. B 110(37) , 18641-50, (2006)] |
|
Dynamic properties of interaction between nucleic acid bases...
1989-01-01 [Biochem. Int. 18(1) , 189-95, (1989)] |
|
Fourier transform infrared spectroscopy of 1-cyclohexyluraci...
2009-03-28 [J. Chem. Phys. 130(12) , 125102, (2009)] |
|
Orotic acid decarboxylation in water and nonpolar solvents: ...
2009-09-15 [Biochemistry 48(36) , 8738-45, (2009)] |
|
Heteroassociation of O- and N-isopropyl derivatives of barbi...
1985-06-01 [Chem. Biol. Interact. 54(1) , 117-25, (1985)] |