Dynamic properties of interaction between nucleic acid bases and models of amino acids.
B S Yu, D H Sohn, G Goo
文献索引:Biochem. Int. 18(1) , 189-95, (1989)
全文:HTML全文
摘要
We have discussed the possible hydrogen bonding mode of adenine-uracil base dimers and peptide side chains, together with their characteristic effects on the stability of adenine-uracil base pairing in chloroform solution by using proton nuclear magnetic resonance methods, where acetamide and butyric acid were used as a model of residues of glutamine and glutamic acid, respectively, and were added to an adenine-uracil equimolar mixture. The stability of base pairing was affected significantly when the model compounds approached the adenine-uracil base pairs; that is, the Hoogsteen type was destroyed, while the Watson-Crick type was formed more favorably.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
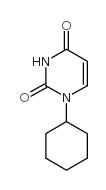 |
1-环己基尿嘧啶
CAS:712-43-6 |
C10H14N2O2 |
|
Solvent-dependent photophysics of 1-cyclohexyluracil: ultraf...
2006-09-21 [J. Phys. Chem. B 110(37) , 18641-50, (2006)] |
|
Fourier transform infrared spectroscopy of 1-cyclohexyluraci...
2009-03-28 [J. Chem. Phys. 130(12) , 125102, (2009)] |
|
Orotic acid decarboxylation in water and nonpolar solvents: ...
2009-09-15 [Biochemistry 48(36) , 8738-45, (2009)] |
|
Heteroassociation of O- and N-isopropyl derivatives of barbi...
1985-06-01 [Chem. Biol. Interact. 54(1) , 117-25, (1985)] |
|
Effect of protein side chain amide group on the hydrogen-bon...
1989-09-15 [Biophys. Chem. 34(1) , 1-8, (1989)] |