| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
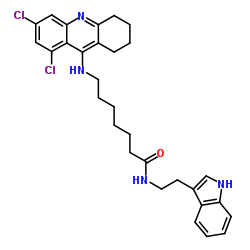 |
酰化酶
CAS:9012-37-7 |
|
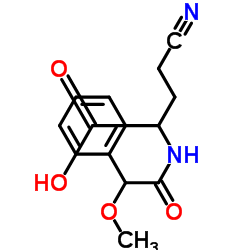 |
辛基琼脂糖凝胶4FF
CAS:68652-09-5 |
|
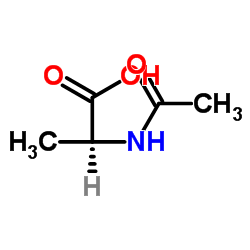 |
N-乙酰-L-丙氨酸
CAS:97-69-8 |