Comparison of intermittent and continuous extracorporeal treatments for the enhanced elimination of dabigatran.
Josée Bouchard, Marc Ghannoum, Amélie Bernier-Jean, David Williamson, Geoffrey Kershaw, Claire Weatherburn, Josette M Eris, Huyen Tran, Jignesh P Patel, Darren M Roberts
文献索引:Clin. Toxicol. (Phila.) 53(3) , 156-63, (2015)
全文:HTML全文
摘要
Severe bleeding associated with dabigatran frequently requires intensive care management. An antidote is currently unavailable and data reporting the effect of dialysis on elimination of dabigatran are encouraging, but limited. Objective. To report the effect of intermittent hemodialysis (IHD) and continuous renal replacement therapy (CRRT) at enhancing elimination of dabigatran.Patients were identified by existing collaborative networks. Pre-filter dabigatran plasma concentrations were measured in all patients, and in dialysate of three patients.Seven patients received dialysis, five with active bleeding and two requiring emergent surgery. Five received IHD and two received CRRT. The plasma elimination half-life of dabigatran was 1.5-4.9 h during IHD, and 14.0-27.5 h during CRRT. Mean dabigatran plasma clearance during IHD was 85-169 mL/min in three patients. Time to obtain a subtherapeutic dabigatran concentration depended on the initial concentration, being 8-18 h for IHD in three patients while 4 h was insufficient in a supratherapeutic case. A 38% rebound in dabigatran levels occurred after one case during IHD, and thrombin time increased after IHD in another, but not after 144 h CRRT or 17 h IHD in two others; data were incomplete in three cases. The amount removed during IHD was proportional to the pre-IHD concentration and clearance, but was consistently low at 3.3-17.4 mg in three patients where this was determined. Moderate bleeding occurred while obtaining vascular access in one patient. Two patients died from intracerebral bleeding, and the influence of treatments could not be determined in these cases.IHD enhanced elimination of dabigatran more efficiently than CRRT, but their net effect remains poorly defined. Dialysis decisions, including modality and duration, must be individualized based on a risk-benefit assessment.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
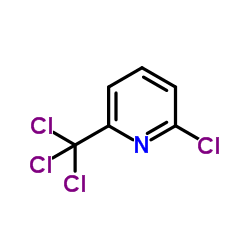 |
氯草定
CAS:1929-82-4 |
C6H3Cl4N |
|
Reductive dehalogenation of the trichloromethyl group of nit...
1993-11-01 [Appl. Environ. Microbiol. 59(11) , 3597-601, (1993)] |
|
Selective enhancement of the fluorescent pseudomonad populat...
2008-10-01 [Microb. Ecol. 56(3) , 538-54, (2008)] |
|
Teratologic evaluation of orally administered nitrapyrin in ...
1988-10-01 [Fundam. Appl. Toxicol. 11(3) , 464-71, (1988)] |
|
Distribution of nitrapyrin [12-chloro-6-(trichloromethyl)-py...
1982-05-01 [J. Sci. Food Agric. 33(5) , 451-5, (1982)] |
|
Nitrapyrin: a scientific advisory group review of the mode o...
2008-06-01 [Regul Toxicol Pharmacol 51(1) , 53-65, (2008)] |