Teratologic evaluation of orally administered nitrapyrin in rats and rabbits.
N M Berdasco, L G Lomax, M A Zimmer, T R Hanley
文献索引:Fundam. Appl. Toxicol. 11(3) , 464-71, (1988)
全文:HTML全文
摘要
Pregnant Fischer 344 rats and New Zealand White rabbits were orally administered 0, 5, 15, or 50 mg nitrapyrin/kg/day on Gestation Days 6 through 15 (rats) or 0, 3, 10, or 30 mg/kg/day on Gestation Days 6 through 18 (rabbits). In rats, 50 mg/kg/day produced slight histopathologic changes in the livers of pregnant females. Fetal examination revealed no evidence of fetotoxicity or teratogenicity among rats at dose levels up to 50 mg/kg/day. Among rabbits, a significant depression in maternal weight gain and increased absolute and relative liver weights were observed at 30 mg/kg/day. An increased incidence of crooked hyoid bone among fetal rabbits in the 30 mg/kg/day dose group was considered indicative of fetotoxicity but not teratogenicity. Thus, administration of nitrapyrin was not teratogenic at dose levels up to 50 mg/kg/day in rats and 30 mg/kg/day in rabbits.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
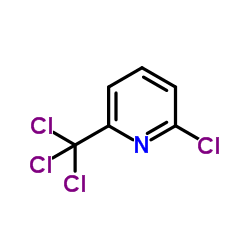 |
氯草定
CAS:1929-82-4 |
C6H3Cl4N |
|
Reductive dehalogenation of the trichloromethyl group of nit...
1993-11-01 [Appl. Environ. Microbiol. 59(11) , 3597-601, (1993)] |
|
Selective enhancement of the fluorescent pseudomonad populat...
2008-10-01 [Microb. Ecol. 56(3) , 538-54, (2008)] |
|
Distribution of nitrapyrin [12-chloro-6-(trichloromethyl)-py...
1982-05-01 [J. Sci. Food Agric. 33(5) , 451-5, (1982)] |
|
Nitrapyrin: a scientific advisory group review of the mode o...
2008-06-01 [Regul Toxicol Pharmacol 51(1) , 53-65, (2008)] |
|
Construction of a highly bioluminescent Nitrosomonas as a pr...
1999-07-01 [Arch. Microbiol. 172(1) , 45-50, (1999)] |