Non-cholinergic synaptic excitation in neostriatum: pharmacological evidence for mediation by a glutamate-like transmitter.
G E Cordingley, F F Weight
文献索引:Br. J. Pharmacol. 88(4) , 847-56, (1986)
全文:HTML全文
摘要
We studied the synaptic pharmacology of an excitatory pathway in the neostriatum using electrophysiological techniques in tissue slices from rats. In response to single electrical stimuli, two negative, extracellular potentials (N-1 and N-2) were recorded through micropipette electrodes within 150-450 micron of the stimulating cathode. N-2 was reversibly reduced or abolished by reducing the concentration of calcium in the bathing medium, while N-1 was unaffected. Both N-1 and N-2 were reversibly abolished by the local anaesthetic procaine. Single-unit, extracellular action potentials were, at times, associated with either N-1 or N-2. Intracellular recordings showed action potentials at N-2 latency arising from graded, monophasic, depolarizing potentials. Bath-applied cholinoceptor and dopamine receptor antagonists failed to reduce N-2. By contrast, antagonists of excitatory amino acid transmitters reversibly reduced or abolished N-2. gamma-D-Glutamylglycine (GG), (+/-)-cis-2,3-piperidine dicarboxylic acid (PDA) and DL-2-amino-4-phosphonobutyric acid (APB) blocked N-2 with ED50S of 0.79 mM, 1.0 mM and 1.1 mM, respectively. (-)-Baclofen reversibly blocked N-2 with an ED50 of 0.79 microM; (+)-baclofen was 330 times less potent. The results suggest that N-1 results from direct activation of fibre tracts or cell bodies, while N-2 is a population spike mediated by excitatory synapses whose natural transmitter pharmacologically resembles glutamate.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
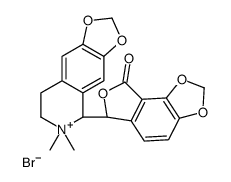 |
(-)-双甲基溴甲烷
CAS:66016-70-4 |
C21H20BrNO6 |
|
Correlated changes in NMDA receptor phosphorylation, functio...
2010-12-01 [J. Neurochem. 115(5) , 1112-22, (2010)] |
|
Further characterization of the anorexic action of orally-ad...
1982-11-01 [Physiol. Behav. 29(5) , 901-4, (1982)] |
|
Increased c-Fos expression in select lateral parabrachial su...
2003-06-06 [Brain Res. 974(1-2) , 153-66, (2003)] |
|
The 4-aminopyridine in vitro epilepsy model analyzed with a ...
2011-06-01 [Neuropharmacology 60(7-8) , 1142-53, (2011)] |
|
Origin of synchronized oscillations induced by neocortical d...
2000-12-15 [J. Neurosci. 20(24) , 9195-206, (2000)] |