Synthesis and evaluation of novel multimeric neurotensin(8-13) analogs.
Christina Hultsch, Beate Pawelke, Ralf Bergmann, Frank Wuest
文献索引:Bioorg. Med. Chem. 14(17) , 5913-20, (2006)
全文:HTML全文
摘要
Neurotensin(8-13) is a hexapeptide with subnanomolar affinity to the neurotensin receptor 1 which is expressed with high incidence in several human tumor entities. Thus, radiolabeled neurotensin(8-13) might be used for tumor targeting. However, its application is limited by insufficient metabolic stability. The present study aims at improving metabolic stability by the synthesis of multimeric neurotensin(8-13) derivatives rather than commonly employed chemical modifications of the peptide itself. Thus, different dimeric and tetrameric peptides carrying C- or N-terminal attached neurotensin(8-13) moieties have been synthesized and their binding affinity toward the neurotensin receptor has been determined. The results demonstrate that branched compounds containing neurotensin(8-13) attached via its C-terminus only show low receptor affinities, whilst derivatives with neurotensin(8-13) attached via the N-terminus show IC50 values in the nanomolar range. Moreover, within the multimeric neurotensin(8-13) derivatives with neurotensin(8-13) attached via the N-terminus an increasing number of branching units lead to higher binding affinities toward the neurotensin receptor.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
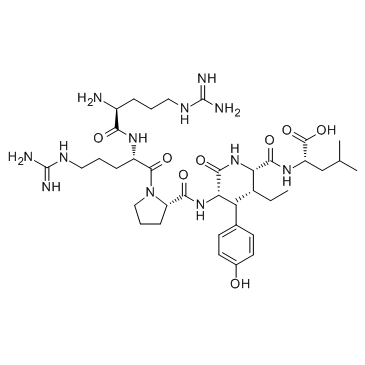 |
神经降压素(8-13)
CAS:60482-95-3 |
C38H64N12O8 |
|
In vivo behavioral effects of stable, receptor-selective neu...
2005-03-01 [Neuropharmacology 48(3) , 417-25, (2005)] |
|
The neurotensin fragment AcNT(8-13) inhibits lowering of int...
2002-09-01 [Am. J. Physiol. Heart Circ. Physiol. 283(3) , H933-40, (2002)] |
|
Radiolabeling of multimeric neurotensin(8-13) analogs with t...
2007-07-01 [Appl. Radiat. Isot. 65(7) , 818-26, (2007)] |
|
Gq/11-induced and spontaneous waves of coordinated network a...
2005-02-16 [J. Neurosci. 25(7) , 1737-49, (2005)] |
|
Radionuclide imaging of small-cell lung cancer (SCLC) using ...
2006-05-01 [Nucl. Med. Biol. 33(4) , 505-12, (2006)] |