Novel bUT-B2 urea transporter isoform is constitutively activated.
P Tickle, A Thistlethwaite, C P Smith, G S Stewart
文献索引:Am. J. Physiol. Regul. Integr. Comp. Physiol. 297(2) , R323-9, (2009)
全文:HTML全文
摘要
Our previous studies have detailed a novel facilitative UT-B urea transporter isoform, bUT-B2. Despite the existence of mouse and human orthologs, the functional characteristics of UT-B2 remain undefined. In this report, we produced a stable MDCK cell line that expressed bUT-B2 protein and investigated the transepithelial urea flux across cultured cell monolayers. We observed a large basal urea flux that was significantly reduced by known inhibitors of facilitative urea transporters; 1,3 dimethylurea (P < 0.001, n = 17), thionicotinamide (P < 0.05, n = 11), and phloretin (P < 0.05, n = 9). Pre-exposure for 1 h to the antidiuretic hormone vasopressin had no effect on bUT-B2-mediated urea transport (NS, n = 3). Acute vasopressin exposure for up to 30 min also failed to elicit any transient response (NS, n = 9). Further investigation confirmed that bUT-B2 function was not affected by alteration of intracellular cAMP (NS, n = 4), intracellular calcium (NS, n = 3), or protein kinase activity (NS, n = 4). Finally, immunoblot data suggested a possible role for glycosylation in regulating bUT-B2 function. In conclusion, this study showed that bUT-B2-mediated transepithelial urea transport was constitutively activated and unaffected by known regulators of renal UT-A urea transporters.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
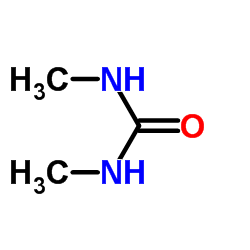 |
1,3-二甲基脲
CAS:96-31-1 |
C3H8N2O |
|
Differential protein abundance and function of UT-B urea tra...
2010-03-01 [Am. J. Physiol. Gastrointest. Liver Physiol. 298(3) , G345-51, (2010)] |
|
In vitro stimulation by paraquat of reactive oxygen-mediated...
1981-09-15 [Toxicol. Appl. Pharmacol. 60(2) , 279-86, (1981)] |
|
Unfolding free energy changes determined by the linear extra...
1988-10-18 [Biochemistry 27(21) , 8063-8, (1988)] |
|
Acute regulation of mUT-A3 urea transporter expressed in a M...
2007-04-01 [Am. J. Physiol. Renal Physiol. 292(4) , F1157-63, (2007)] |
|
Multiple hydrogen-bonding interactions between macrocyclic t...
2011-04-28 [Phys. Chem. Chem. Phys. 13(16) , 7384-95, (2011)] |